Technology
Gylden are developing an extensive range of T cell-priming vaccines and immunotherapy candidates for infectious diseases and other indications using core proprietary technologies designed to elicit broad and long-lasting cellular immune responses.
Gylden product candidates are comprised of the core technologies described below:
- Immunoproteomics and Multi-Omic Technologies
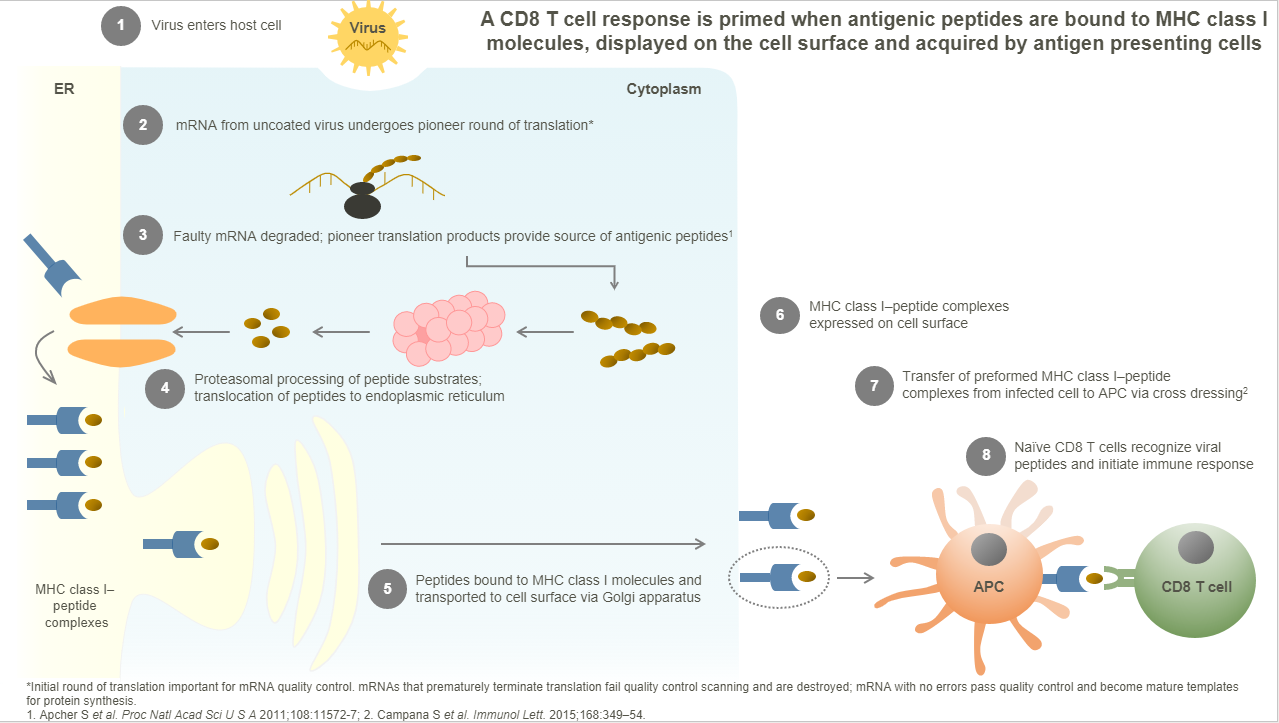
- An MHC Class I expression ‘ligandome library’ of pathogen-derived protein fragments (peptides) are experimentally-determined to be expressed on the surface of pathogen-infected cells and therefore represent the targets of the T cell response during an infection.
- Nanotechnology Particles: An ultrasmall carbohydrate-passivated nanocluster delivery system
- Fully synthetic peptides are designed to be efficiently delivered to the skin-resident immune system to elicit a robust T cell immune response.
- Microneedle Array Patch [MAP] Technology
- Advanced delivery and R&D assets were acquired via the purchase of Zosano Pharma in 2022. Preliminary studies were completed that combine Zosano MAP technology with our T cell-priming candidates, consolidating the platform’s potential and supporting the development of our many other T cell-priming candidates.
- In-house GMP Nanomedicine Manufacturing Capabilities
- Issued by the UK’s MHRA, the Company received its MIA(IMP) license and cGMP accreditation in 2024.
With Gylden’s product candidates, viral peptides are presented to T cells as in the natural immune response.
- Gylden’s approach is designed to deliver fully synthetic viral peptides to antigen presenting cells (eg. dendritic cells ), activating naïve T cells with specificity to a virus that are then expanded to a memory phenotype.
- Such virus-specific memory CD8+ T cells have the potential to migrate to tissue sites of potential pathogen exposure following a second vaccination.
- A pre-existing population of virus-specific CD8+ T cells is thus created that, upon subsequent pathogen encounter, would be able to immediately kill infected cells before they can release virions, and the infection is rapidly terminated.
An approach designed to mimic natural immunity
Our goal is to create a critical mass of ‘primed’ tissue-resident memory CD8+ T cells and shorter-lived CD8+ T effector cells with specificities to early pathogen peptides, equipped to provide broad and long-lasting cellular immunity upon subsequent infection. T cell adaptive vaccines evoke the course of cellular immunity that is prompted through natural infection but halt the infection locally — at the site of transmission — before progression to full-blown disease would take place.
Product candidates designed to prime the immune system to enable a rapid response to future infection
The adaptive immune response to an infection includes CD8+ T cells that recognize pathogen-derived peptides (below, from a viral infection) associated with MHC Class I molecules expressed on the surface of infected cells.